Difference between revisions of "DHEA"
| (16 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | Dehydroepiandrosterone is a steroid hormone, a chemical cousin of testosterone and estrogen.<br /><br />Study reported in 2006 in <span title="Clinical endocrinology.">Clin Endocrinol. </span>shows that short-term treatment with DHEA increased platelet cGMP production, a marker of NO production, in healthy elderly subjects. This effect is coupled with a decrease in PAI-1 and LDL cholesterol levels as well as an increase in testosterone and E(2) levels. These findings, therefore, suggest that chronic DHEA supplementation would exert antiatherogenic effects, particularly in elderly subjects who display low circulating levels of this hormone.<br /><br /><br />[http://www.pubmed.org Pubmed] | [[Vitamin]]<br /> | + | Dehydroepiandrosterone is a steroid hormone, a chemical cousin of [[testosterone]] and [[estrogen]].<br /> |
| + | <br /> | ||
| + | Study reported in 2006 in <span title="Clinical endocrinology.">Clin Endocrinol. </span>shows that short-term treatment with DHEA increased platelet cGMP production, a marker of NO production, in healthy elderly subjects. This effect is coupled with a decrease in PAI-1 and LDL cholesterol levels as well as an increase in testosterone and E(2) levels. These findings, therefore, suggest that chronic DHEA supplementation would exert antiatherogenic effects, particularly in elderly subjects who display low circulating levels of this hormone.<br /> | ||
| + | <br /> | ||
| + | Studies have shown that DHEA inhibits carcinogenesis in mammary gland and prostate as well as other organs, a process that is not hormone dependent (2006)<br /> | ||
| + | <br /> | ||
| + | Although the analysis is limited by the short follow-up and small number of deaths, results are consistent with the notion that DHEAS level has a sizeable effect on mortality. (2006)<br /> | ||
| + | <br /> | ||
| + | <p><strong>Dehydroepiandrosterone</strong> (<strong>DHEA</strong>), is a natural steroid prohormone produced from cholesterol by the adrenal glands, the gonads, adipose tissue, brain and in the skin (by an [[autocrine mechanism]])]. DHEA is the precursor of androstenedione, testosterone and estrogen. It is the most abundant hormone in the human body.<br /> | ||
| + | <table id="drugInfoBox" style="CLEAR: right; BACKGROUND: #ffffff; FLOAT: right; MARGIN: 0px 0px 0.5em 1em" cellpadding="1" width="280" align="right" border="0" class="toccolours"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td align="center" colspan="2"><a class="image" title="" href="http://en.wikipedia.org/wiki/Image:Dehydroepiandrosterone.png"><img height="118" alt="" width="177" longdesc="/wiki/Image:Dehydroepiandrosterone.png" src="http://upload.wikimedia.org/wikipedia/en/9/98/Dehydroepiandrosterone.png" /></a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align="center" colspan="2"> | ||
| + | <div style="FONT-SIZE: medium; LINE-HEIGHT: 167%">Dehydroepiandrosterone</div> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#dddddd" colspan="2"><strong>Systematic (<a title="International Union of Pure and Applied Chemistry nomenclature" href="http://en.wikipedia.org/wiki/International_Union_of_Pure_and_Applied_Chemistry_nomenclature">IUPAC</a>) name</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="VERTICAL-ALIGN: top; TEXT-ALIGN: center" bgcolor="#eeeeee" colspan="2"><span style="FONT-SIZE: 11px">3ß-hydroxy-5-androsten-17-one</span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#dddddd" colspan="2"><strong>Identifiers</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td width="90" bgcolor="#ddeeff"><a title="CAS registry number" href="http://en.wikipedia.org/wiki/CAS_registry_number">CAS number</a></td> | ||
| + | <td bgcolor="#eeeeee"><span class="reflink plainlinksneverexpand"><a class="external text" title="http://www.nlm.nih.gov/cgi/mesh/2006/MB_cgi?term=53-43-0&rn=1" rel="nofollow" href="http://www.nlm.nih.gov/cgi/mesh/2006/MB_cgi?term=53-43-0&rn=1">53-43-0</a></span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#ddeeff"><a title="Anatomical Therapeutic Chemical Classification System" href="http://en.wikipedia.org/wiki/Anatomical_Therapeutic_Chemical_Classification_System">ATC code</a></td> | ||
| + | <td bgcolor="#eeeeee"><a title="ATC code A14" href="http://en.wikipedia.org/wiki/ATC_code_A14">A14</a><span class="reflink plainlinksneverexpand"><a class="external text" title="http://www.whocc.no/atcddd/indexdatabase/index.php?query=A14AA07" rel="nofollow" href="http://www.whocc.no/atcddd/indexdatabase/index.php?query=A14AA07">AA07</a></span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#ddeeff"><a title="PubChem" href="http://en.wikipedia.org/wiki/PubChem">PubChem</a></td> | ||
| + | <td bgcolor="#eeeeee"><span class="reflink plainlinksneverexpand"><a class="external text" title="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=76" rel="nofollow" href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=76">76</a></span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#dddddd" colspan="2"><strong>Chemical data</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#ddeeff"><a title="Chemical formula" href="http://en.wikipedia.org/wiki/Chemical_formula">Formula</a></td> | ||
| + | <td bgcolor="#eeeeee"><a title="Carbon" href="http://en.wikipedia.org/wiki/Carbon"><span style="FONT-WEIGHT: bold; COLOR: rgb(0,0,0)">C</span></a><sub>19</sub><a title="Hydrogen" href="http://en.wikipedia.org/wiki/Hydrogen"><span style="FONT-WEIGHT: bold; COLOR: rgb(77,77,77)">H</span></a><sub>28</sub><a title="Oxygen" href="http://en.wikipedia.org/wiki/Oxygen"><span style="FONT-WEIGHT: bold; COLOR: rgb(116,35,35)">O</span></a><sub>2</sub><sup> </sup></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#ddeeff"><a title="Molecular mass" href="http://en.wikipedia.org/wiki/Molecular_mass">Mol. mass</a></td> | ||
| + | <td bgcolor="#eeeeee">288.43</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#dddddd" colspan="2"><strong>Physical data</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#ddeeff"><a title="Melting point" href="http://en.wikipedia.org/wiki/Melting_point">Melt. point</a></td> | ||
| + | <td bgcolor="#eeeeee">148.5 °C (299 °F)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#dddddd" colspan="2"><strong>Pharmacokinetic data</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="VERTICAL-ALIGN: top" bgcolor="#ddeeff"><a title="Bioavailability" href="http://en.wikipedia.org/wiki/Bioavailability">Bioavailability</a></td> | ||
| + | <td bgcolor="#eeeeee"> ?</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="VERTICAL-ALIGN: top" bgcolor="#ddeeff"><a title="Drug metabolism" href="http://en.wikipedia.org/wiki/Drug_metabolism">Metabolism</a></td> | ||
| + | <td bgcolor="#eeeeee"><a title="Liver" href="http://en.wikipedia.org/wiki/Liver">Hepatic</a></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="VERTICAL-ALIGN: top" bgcolor="#ddeeff"><a title="Biological half-life" href="http://en.wikipedia.org/wiki/Biological_half-life">Half life</a></td> | ||
| + | <td bgcolor="#eeeeee">12 hours</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="VERTICAL-ALIGN: top" bgcolor="#ddeeff"><a title="Excretion" href="http://en.wikipedia.org/wiki/Excretion">Excretion</a></td> | ||
| + | <td bgcolor="#eeeeee"><a title="Urine" href="http://en.wikipedia.org/wiki/Urine">Urinary</a>:?%</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td bgcolor="#dddddd" colspan="2"><strong>Therapeutic considerations</strong></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="VERTICAL-ALIGN: top" bgcolor="#ddeeff"><a title="Pregnancy category" href="http://en.wikipedia.org/wiki/Pregnancy_category">Pregnancy cat.</a></td> | ||
| + | <td bgcolor="#eeeeee"> | ||
| + | <p>?</p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="VERTICAL-ALIGN: top" bgcolor="#ddeeff"><a title="Regulation of therapeutic goods" href="http://en.wikipedia.org/wiki/Regulation_of_therapeutic_goods">Legal status</a></td> | ||
| + | <td bgcolor="#eeeeee"> | ||
| + | <p>Commercially available<br /> | ||
| + | (<a title="United States" href="http://en.wikipedia.org/wiki/United_States">US</a>)</p> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="VERTICAL-ALIGN: top" bgcolor="#ddeeff"><a title="Route of administration" href="http://en.wikipedia.org/wiki/Route_of_administration">Routes</a></td> | ||
| + | <td bgcolor="#eeeeee"><a title="Mouth" href="http://en.wikipedia.org/wiki/Mouth">Oral</a></td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | </p> | ||
| + | <p><span class="mw-headline"><font size="5"><br /> | ||
| + | Synonyms and brand names</font></span></p> | ||
| + | <p>Synonyms for Dehydroepiandrosterone are: Dehydroisoandrosterone; 3β-Hydroxy-5-androsten-17-one; 3β-Hydroxyandrost-5-en-17-one; Androstenol; Androstenolone; Dehydroisoandrosterone; Hydroxyandrost-5-en-17-one; Prasterone; trans-Dehydroandrosterone.</p> | ||
| + | <p>Brand names for DHEA include Prastera® and Fidelin®.</p> | ||
| + | <p> </p> | ||
| + | <p><span class="mw-headline"><font size="5"><br /> | ||
| + | DHEAS (Dehydroepiandrosterone sulfate)</font></span></p> | ||
| + | <p><strong>Dehydroepiandrosterone sulfate</strong> (<strong>DHEAS</strong>, PubChem 12594) is the sulfated version of DHEA, - this conversion is reversibly catalyzed by sulfotransferase (SULT2A1) primarily in the adrenals, the liver, and small intestines. In blood, most DHEA is found as DHEAS with levels that are about 300 times higher than free DHEA. Orally ingested DHEA is converted to its sulfate when passing through intestines and liver. While DHEA levels reach their peak in the early morning hours, DHEAS levels show no diurnal variation.</p> | ||
| + | <p>From a practical point measurement of DHEAS is preferable to DHEA as levels are more stable.</p> | ||
| + | <p> </p> | ||
| + | <p><span class="mw-headline"><font size="5"><br /> | ||
| + | Production</font></span></p> | ||
| + | <div class="thumb tright"> | ||
| + | <div class="thumbinner" style="WIDTH: 302px"><img class="thumbimage" height="121" alt="Production of DHEA from Cholesterol" width="300" longdesc="/wiki/Image:DHEA1.svg" src="http://upload.wikimedia.org/wikipedia/commons/thumb/6/6c/DHEA1.svg/300px-DHEA1.svg.png" /> | ||
| + | <div class="thumbcaption"> | ||
| + | <div class="magnify" style="FLOAT: right"><img height="11" alt="" width="15" src="http://en.wikipedia.org/skins-1.5/common/images/magnify-clip.png" /></div> | ||
| + | Production of DHEA from Cholesterol</div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <p>DHEA is produced from cholesterol through two cytochrome P450 enzymes. Cholesterol is converted to pregnenolone by the enzyme P450 scc (side chain cleavage) and then another enzyme CYP17A1 converts pregnenolone to 17α-Hydroxypregnenolone and then to DHEA. In humans DHEA is the dominant steroid hormone and precursor of all sex steroids. Humans produce DHEA in greater quantity than any other species. Even non-human primates have not much more than 10% the relative serum level of DHEA seen in humans. The fact that rodents produce so little DHEA makes the results of experiments conducted with these laboratory animals very controversial.</p> | ||
| + | <p>DHEA production is very high during fetal life by the fetal adrenal glands, declines after birth and remains low during childhood. Production begins around 6 years of age, increasing in quantity until peaking in early adulthood, around the age of 25, and declines afterwards to approximately 10% of peak levels by age 80. It is theorized by some that this decline may be due to reduced oxygen and glucose supply to the adrenal glands as a result of age-related atherosclerosis.</p> | ||
| + | <p> </p> | ||
| + | <p><span class="mw-headline"><font size="5">Role</font></span></p> | ||
| + | <p>In a simple view DHEA can be understood as a prohormone for the sex steroids. Its DHEAS variation may be looked at as buffer and reservoir. Its production in the brain suggests that it also has a role as a neurosteroid. As most DHEA is produced by the zona reticularis of the adrenal, it is argued that there is a role in the immune and stress response. DHEA may have more biologic roles.</p> | ||
| + | <p>As almost all DHEA is derived from the adrenal glands, blood measurements of DHEAS/DHEA are useful to detect excess adrenal activity as seen in adrenal cancer or hyperplasia, including certain forms of congenital adrenal hyperplasia. Women with polycystic ovary syndrome tend to have normal or mildly elevated levels of DHEAS.</p> | ||
| + | <p> </p> | ||
| + | <p><span class="mw-headline"><font size="5"><br /> | ||
| + | Beneficial Effects</font></span></p> | ||
| + | <p>Studies have shown that DHEA is useful in patients with systemic lupus erythematosus. An application of the evidence was reviewed by the FDA in 2001 and is available online.<sup class="reference" id="_ref-0">[1]</sup> This review also shows that cholesterol and other serum lipids decrease with the use of DHEA (mainly a decrease in HDL-C and triglycerides can be expected in women, p110).</p> | ||
| + | <p>Supplementation with DHEA has been shown to decrease insulin resistance.<sup class="reference" id="_ref-1">[2]</sup></p> | ||
| + | <p>Long term supplementation has been shown to improve mood and relieve depression.<sup class="reference" id="_ref-2">[3]</sup></p> | ||
| + | <p>It is suggested that DHEA's beneficial effects arise from its antioxidant, antiobesity, antilipofuscin, antilipidperoxidative and thereby anti-aging actions. <span title="Biogerontology."><a href="javascript:AL_get(this, 'jour', 'Biogerontology.');">Biogerontology.</a></span> 2008 Feb 29<br /> | ||
| + | </p> | ||
| + | <p><span class="mw-headline"><font size="4">Disputed effects</font></span></p> | ||
| + | <p>Hormonal substitution with DHEA, testosterone, and growth hormone is ineffective as anti-aging medicine, while calcium and vitamin d'effectiveness was confirmed in fracture prevention. <span title="Revue médicale suisse."><a href="javascript:AL_get(this, 'jour', 'Rev Med Suisse.');">Rev Med Suisse.</a></span> 2008 Jan 9;4(139):18-20, 22-3<br /> | ||
| + | <br /> | ||
| + | The significance of the hormone in health and disease is not fully established. It is postulated that DHEA supplements are beneficial in alleviating:</p> | ||
| + | <ul> | ||
| + | <li>cardiovascular disease </li> | ||
| + | <li>diabetes </li> | ||
| + | <li>hypercholesterolemia </li> | ||
| + | <li>obesity </li> | ||
| + | <li>multiple sclerosis </li> | ||
| + | <li>Parkinson's disease </li> | ||
| + | <li>Alzheimer's disease </li> | ||
| + | <li>disorders of the immune system </li> | ||
| + | <li>depression </li> | ||
| + | <li>osteoporosis </li> | ||
| + | <li>decreased libido </li> | ||
| + | <li>decreased orgasmic intensity </li> | ||
| + | </ul> | ||
| + | <p>It is also commercially advertised that DHEA:</p> | ||
| + | <ul> | ||
| + | <li>helps decrease insulin resistance </li> | ||
| + | <li>improves fat metabolism </li> | ||
| + | <li>increases immune system function </li> | ||
| + | <li>has anti-aging properties </li> | ||
| + | <li>increases lean muscle mass </li> | ||
| + | </ul> | ||
| + | <p>7-Keto™ DHEA, a recently identified metabolite of dehydroepiandrosterone (DHEA) is claimed to be both more effective and safer than DHEA because it does not convert itself into testosterone or estrogens in the body.<sup class="noprint Template-Fact"><span title="This claim needs references to reliable sources since February 2007" style="WHITE-SPACE: nowrap">[<em>citation needed</em>]</span></sup></p> | ||
| + | <p>DHEA and DHEAS are readily available in the United States.</p> | ||
| + | <p> </p> | ||
| + | <p><span class="mw-headline"><font size="5">Precautions</font></span></p> | ||
| + | <p>Some assert that DHEA should not be supplemented outside specialist centres under careful observation of experts in the field of endocrinology.</p> | ||
| + | <p>Side effects may include:</p> | ||
| + | <ul> | ||
| + | <li>Palpitations and other arrhythmias </li> | ||
| + | <li>extensive growth of body hair, or hirsutism </li> | ||
| + | <li>Hair loss, especially male pattern baldness </li> | ||
| + | <li>acne </li> | ||
| + | </ul> | ||
| + | <p> </p> | ||
| + | <p><span class="mw-headline"><font size="5">Contraindication</font></span></p> | ||
| + | <p>As DHEAS and DHEA are converted to sex steroids, their use is contraindicated in patients with any cancer that is estrogen or testosterone dependent.<sup class="noprint Template-Fact"><span title="This claim needs references to reliable sources since February 2007" style="WHITE-SPACE: nowrap">[<em>citation needed</em>]</span></sup></p> | ||
| + | <p> </p> | ||
| + | <p><span class="mw-headline"><font size="5">Increasing endogenous production</font></span></p> | ||
| + | <p>Regular exercise is known to increase DHEA production in the body.<sup class="reference" id="_ref-3">[4]</sup><sup class="reference" id="_ref-4">[5]</sup><sup class="reference" id="_ref-5">[6]</sup> Caloric restriction has also been shown to increase DHEA in primates.<sup class="reference" id="_ref-6">[7]</sup></p> | ||
| + | <p> </p> | ||
| + | <h2><span class="mw-headline">See also</span></h2> | ||
| + | <ul> | ||
| + | <li>Steroid hormones </li> | ||
| + | <li>Testosterone </li> | ||
| + | <li>Estrogen </li> | ||
| + | </ul> | ||
| + | <p> </p> | ||
| + | <h2><span class="mw-headline">References</span></h2> | ||
| + | <ol class="references"> | ||
| + | <li id="_note-0"><strong>^</strong> FDA document regading DHEA and SLE </li> | ||
| + | <li id="_note-1"><strong>^</strong> Kawano H, Yasue H, Kitagawa A, et al. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5. </li> | ||
| + | <li id="_note-2"><strong>^</strong> Wolkowitz OM, Reus VI, Roberts E, et al. Antidepressant and cognition-enhancing effects of DHEA in major depression. Ann NY Acad Sci. 1995 Dec 29;774:337-9 </li> | ||
| + | <li id="_note-3"><strong>^</strong> Eur J Appl Physiol Occup Physiol 1998 Oct;78(5):466-71 </li> | ||
| + | <li id="_note-4"><strong>^</strong> Eur J Appl Physiol. 2001 Jul;85(1- 2):177-84 </li> | ||
| + | <li id="_note-5"><strong>^</strong> J Gerontol A Biol Sci Med Sci. 2002 Apr;57(4):B158-65 </li> | ||
| + | <li id="_note-6"><strong>^</strong> Exp Gerontol. 2003 Jan-Feb; 38(1-2):35-46 </li> | ||
| + | </ol> | ||
| + | <p><a id="Further_reading" name="Further_reading"></a></p> | ||
| + | <h2><span class="mw-headline">Further reading</span></h2> | ||
| + | <ul> | ||
| + | <li>Nutrition through the Life Cycle, Judith E. Brown, <a class="internal" href="http://en.wikipedia.org/w/index.php?title=Special:Booksources&isbn=0534589863">ISBN 0-534-58986-3</a> </li> | ||
| + | <li>Andus T, et al. <font face="Arial">Patients with refractory Crohn's disease or ulcerative colitis respond to dehydroepiandrosterone: a pilot study. Aliment Pharmacol Ther. 2003 Feb;17(3):409-14.<br /> | ||
| + | <strong>Arlt W, Callies F, Allolio B.</strong> DHEA replacement in women with adrenal insufficiency--pharmacokinetics, bioconversion and clinical effects on well-being, sexuality and cognition. Endocr Res. 2000 Nov;26(4):505-11.<br /> | ||
| + | <strong>Chang DM, et al. </strong>Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002 Nov;46(11):2924-7. <br /> | ||
| + | <strong>Gordon CM, et al. </strong>Effects of oral DHEA on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002 Nov;87(11):4935-41. <br /> | ||
| + | <strong>Hackbert L, Heiman JR.</strong> Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. J Womens Health Gend Based Med. 2002 Mar;11(2):155-62. <br /> | ||
| + | <strong>Hirshman E, et al. </strong>The effect of dehydroepiandrosterone (DHEA) on recognition memory decision processes and discrimination in postmenopausal women. Psychon Bull Rev. 2003 Mar;10(1):125-34. <br /> | ||
| + | <strong>Johannsson G, et al. </strong>Low dose DHEA affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab. 2002 May;87(5):2046-52. <br /> | ||
| + | <strong>Kawano H, et al. </strong>DHEA supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5. <br /> | ||
| + | <strong>Lovas K, et al. </strong>Replacement of DHEA in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003 Mar;88(3):1112-8. <br /> | ||
| + | <strong>Percheron G, et al. </strong>Effect of 1-year oral administration of DHEA to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003 Mar 24;163(6):720-7. <br /> | ||
| + | <strong>Piketty C, et al. </strong>Double-blind placebo-controlled trial of oral DHEA in patients with advanced HIV disease. Clin Endocrinol (Oxf). 2001 Sep;55(3):325-30. <br /> | ||
| + | <strong>Sahelian, R, Borken, S.</strong> DHEA and cardiac arrhythmia. Ann Intern Med. 1998 Oct 1;129(7):588. <br /> | ||
| + | <strong>Sun Y, et al. </strong>Treatment of osteoporosis in men using dehydroepiandrosterone sulfate. Chin Med J (Engl). 2002 Mar;115(3):402-4. <strong><br /> | ||
| + | <br /> | ||
| + | Andus T, et al. </strong>Patients with refractory Crohn's disease or ulcerative colitis respond to DHEA: a pilot study. Aliment Pharmacol Ther. 2003 Feb;17(3):409-14.<br /> | ||
| + | <strong>Arlt W, Callies F, Allolio B.</strong> DHEA replacement in women with adrenal insufficiency--pharmacokinetics, bioconversion and clinical effects on well-being, sexuality and cognition. Endocr Res. 2000 Nov;26(4):505-11.<strong><br /> | ||
| + | Chang DM, et al. DHEA </strong>treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002 Nov;46(11):2924-7. <strong><br /> | ||
| + | Gordon CM, et al. </strong>Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002 Nov;87(11):4935-41. <strong><br /> | ||
| + | Hackbert L, Heiman JR.</strong> Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. J Womens Health Gend Based Med. 2002 Mar;11(2):155-62. <strong><br /> | ||
| + | Hirshman E, et al. </strong>The effect of dehydroepiandrosterone (DHEA) on recognition memory decision processes and discrimination in postmenopausal women. Psychon Bull Rev. 2003 Mar;10(1):125-34. <strong><br /> | ||
| + | Johannsson G, et al. </strong>Low dose DHEA affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab. 2002 May;87(5):2046-52. <strong><br /> | ||
| + | Kawano H, et al. DHEA </strong>supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5. <strong><br /> | ||
| + | Lovas K, et al. </strong>Replacement of DHEA in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003 Mar;88(3):1112-8. <br /> | ||
| + | <strong>Percheron G, et al. </strong>Effect of 1-year oral administration of DHEA to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003 Mar 24;163(6):720-7. <strong><br /> | ||
| + | Piketty C, et al. </strong>Double-blind placebo-controlled trial of oral DHEA in patients with advanced HIV disease. Clin Endocrinol (Oxf). 2001 Sep;55(3):325-30. <br /> | ||
| + | <strong>Sahelian, R, Borken, S.</strong> DHEA and cardiac arrhythmia. Ann Intern Med. 1998 Oct 1;129(7):588. <strong><br /> | ||
| + | Sun Y, et al. </strong>Treatment of osteoporosis in men using DHEA sulfate. Chin Med J (Engl). 2002 Mar;115(3):402-4. </font></li> | ||
| + | </ul> | ||
| + | <p><a id="External_links" name="External_links"></a></p> | ||
| + | <h2><span class="mw-headline">External links</span></h2> | ||
| + | <ul> | ||
| + | <li><a class="external text" title="http://www.lef.org/magazine/mag2004/mar2004_cover_dhea_01.htm" rel="nofollow" href="http://www.lef.org/magazine/mag2004/mar2004_cover_dhea_01.htm"><em>The DHEA Debate: A critical review of experimental data</em></a> at lef.org (Published 2004){This link is to the web equivalent of an infomercial. <strong>Unreliable sponsored analysis</strong>) </li> | ||
| + | <li><a class="external text" title="http://www.quackwatch.org/01QuackeryRelatedTopics/dhea.html" rel="nofollow" href="http://www.quackwatch.org/01QuackeryRelatedTopics/dhea.html"><em>DHEA: Ignore the Hype</em></a> (Published 1996) at <a title="Quackwatch" href="http://en.wikipedia.org/wiki/Quackwatch">Quackwatch</a> </li> | ||
| + | <li><a class="external text" title="http://www.benbest.com/nutrceut/DHEA.html" rel="nofollow" href="http://www.benbest.com/nutrceut/DHEA.html">DHEA Hormone Replacement at benbest.com</a> </li> | ||
| + | <li><a class="external text" title="http://skepdic.com/dhea.html" rel="nofollow" href="http://skepdic.com/dhea.html">What the Skeptic's Dictionary has to say on DHEA</a> </li> | ||
| + | <li><cite style="FONT-STYLE: normal">Nair K, Rizza R, O'Brien P, Dhatariya K, Short K, Nehra A, Vittone J, Klee G, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall D, Melton L, Smith G, Khosla S, Jensen M (2006). "DHEA in elderly women and DHEA or testosterone in elderly men". <em>N Engl J Med</em> <strong>355</strong> (16): 1647-59. <a class="external" title="http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17050889" href="http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17050889">PMID 17050889</a>.</cite><span class="Z3988" title="ctx_ver=Z39.88-2004%26rft_val_fmt%3Dinfo%3Aofi%2Ffmt%3Akev%3Amtx%3Ajournal&rft.atitle=DHEA+in+elderly+women+and+DHEA+or+testosterone+in+elderly+men&rft.title=N+Engl+J+Med&rft.jtitle=N+Engl+J+Med&rft.date=2006&rft.volume=355&rft.issue=16&rft.au=Nair+K%2C+Rizza+R%2C+O%27Brien+P%2C+Dhatariya+K%2C+Short+K%2C+Nehra+A%2C+Vittone+J%2C+Klee+G%2C+Basu+A%2C+Basu+R%2C+Cobelli+C%2C+Toffolo+G%2C+Dalla+Man+C%2C+Tindall+D%2C+Melton+L%2C+Smith+G%2C+Khosla+S%2C+Jensen+M&rft.pages=1647-59&rft_id=info:pmid/17050889"> </span> <a class="external text" title="http://content.nejm.org/cgi/content/abstract/355/16/1647" rel="nofollow" href="http://content.nejm.org/cgi/content/abstract/355/16/1647">link</a> </li> | ||
| + | <li><font color="#008040" size="2">DHEA Supplement by </font><a href="http://www.raysahelian.com/"><font size="2">Ray Sahelian, M.D.</font></a> <a href="http://www.raysahelian.com/dhea.html">http://www.raysahelian.com/dhea.html</a><br /> | ||
| + | <br /> | ||
| + | [http://whyfiles.org/051fat_fixes/dhea.html Whyfiles]<br /> | ||
| + | [http://www.pubmed.org Pubmed] | [[Vitamin]] | [[디에이치이에이]] <br /> | ||
| + | [http://ligandome.org Ligandome]<br /> | ||
| + | </li> | ||
| + | </ul> | ||
Latest revision as of 21:47, 25 March 2008
Dehydroepiandrosterone is a steroid hormone, a chemical cousin of testosterone and estrogen.
Study reported in 2006 in Clin Endocrinol. shows that short-term treatment with DHEA increased platelet cGMP production, a marker of NO production, in healthy elderly subjects. This effect is coupled with a decrease in PAI-1 and LDL cholesterol levels as well as an increase in testosterone and E(2) levels. These findings, therefore, suggest that chronic DHEA supplementation would exert antiatherogenic effects, particularly in elderly subjects who display low circulating levels of this hormone.
Studies have shown that DHEA inhibits carcinogenesis in mammary gland and prostate as well as other organs, a process that is not hormone dependent (2006)
Although the analysis is limited by the short follow-up and small number of deaths, results are consistent with the notion that DHEAS level has a sizeable effect on mortality. (2006)
Dehydroepiandrosterone (DHEA), is a natural steroid prohormone produced from cholesterol by the adrenal glands, the gonads, adipose tissue, brain and in the skin (by an autocrine mechanism)]. DHEA is the precursor of androstenedione, testosterone and estrogen. It is the most abundant hormone in the human body.
 |
|
|
Dehydroepiandrosterone
|
|
| Systematic (IUPAC) name | |
| 3ß-hydroxy-5-androsten-17-one | |
| Identifiers | |
| CAS number | |
| ATC code | A14 |
| PubChem | |
| Chemical data | |
| Formula | C19H28O2 |
| Mol. mass | 288.43 |
| Physical data | |
| Melt. point | 148.5 °C (299 °F) |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Half life | 12 hours |
| Excretion | Urinary:?% |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Commercially available |
| Routes | Oral |
Synonyms and brand names
Synonyms for Dehydroepiandrosterone are: Dehydroisoandrosterone; 3β-Hydroxy-5-androsten-17-one; 3β-Hydroxyandrost-5-en-17-one; Androstenol; Androstenolone; Dehydroisoandrosterone; Hydroxyandrost-5-en-17-one; Prasterone; trans-Dehydroandrosterone.
Brand names for DHEA include Prastera® and Fidelin®.
DHEAS (Dehydroepiandrosterone sulfate)
Dehydroepiandrosterone sulfate (DHEAS, PubChem 12594) is the sulfated version of DHEA, - this conversion is reversibly catalyzed by sulfotransferase (SULT2A1) primarily in the adrenals, the liver, and small intestines. In blood, most DHEA is found as DHEAS with levels that are about 300 times higher than free DHEA. Orally ingested DHEA is converted to its sulfate when passing through intestines and liver. While DHEA levels reach their peak in the early morning hours, DHEAS levels show no diurnal variation.
From a practical point measurement of DHEAS is preferable to DHEA as levels are more stable.
Production
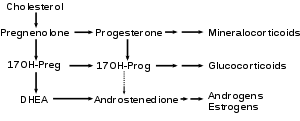
DHEA is produced from cholesterol through two cytochrome P450 enzymes. Cholesterol is converted to pregnenolone by the enzyme P450 scc (side chain cleavage) and then another enzyme CYP17A1 converts pregnenolone to 17α-Hydroxypregnenolone and then to DHEA. In humans DHEA is the dominant steroid hormone and precursor of all sex steroids. Humans produce DHEA in greater quantity than any other species. Even non-human primates have not much more than 10% the relative serum level of DHEA seen in humans. The fact that rodents produce so little DHEA makes the results of experiments conducted with these laboratory animals very controversial.
DHEA production is very high during fetal life by the fetal adrenal glands, declines after birth and remains low during childhood. Production begins around 6 years of age, increasing in quantity until peaking in early adulthood, around the age of 25, and declines afterwards to approximately 10% of peak levels by age 80. It is theorized by some that this decline may be due to reduced oxygen and glucose supply to the adrenal glands as a result of age-related atherosclerosis.
Role
In a simple view DHEA can be understood as a prohormone for the sex steroids. Its DHEAS variation may be looked at as buffer and reservoir. Its production in the brain suggests that it also has a role as a neurosteroid. As most DHEA is produced by the zona reticularis of the adrenal, it is argued that there is a role in the immune and stress response. DHEA may have more biologic roles.
As almost all DHEA is derived from the adrenal glands, blood measurements of DHEAS/DHEA are useful to detect excess adrenal activity as seen in adrenal cancer or hyperplasia, including certain forms of congenital adrenal hyperplasia. Women with polycystic ovary syndrome tend to have normal or mildly elevated levels of DHEAS.
Beneficial Effects
Studies have shown that DHEA is useful in patients with systemic lupus erythematosus. An application of the evidence was reviewed by the FDA in 2001 and is available online.[1] This review also shows that cholesterol and other serum lipids decrease with the use of DHEA (mainly a decrease in HDL-C and triglycerides can be expected in women, p110).
Supplementation with DHEA has been shown to decrease insulin resistance.[2]
Long term supplementation has been shown to improve mood and relieve depression.[3]
It is suggested that DHEA's beneficial effects arise from its antioxidant, antiobesity, antilipofuscin, antilipidperoxidative and thereby anti-aging actions. Biogerontology. 2008 Feb 29
Disputed effects
Hormonal substitution with DHEA, testosterone, and growth hormone is ineffective as anti-aging medicine, while calcium and vitamin d'effectiveness was confirmed in fracture prevention. Rev Med Suisse. 2008 Jan 9;4(139):18-20, 22-3
The significance of the hormone in health and disease is not fully established. It is postulated that DHEA supplements are beneficial in alleviating:
- cardiovascular disease
- diabetes
- hypercholesterolemia
- obesity
- multiple sclerosis
- Parkinson's disease
- Alzheimer's disease
- disorders of the immune system
- depression
- osteoporosis
- decreased libido
- decreased orgasmic intensity
It is also commercially advertised that DHEA:
- helps decrease insulin resistance
- improves fat metabolism
- increases immune system function
- has anti-aging properties
- increases lean muscle mass
7-Keto™ DHEA, a recently identified metabolite of dehydroepiandrosterone (DHEA) is claimed to be both more effective and safer than DHEA because it does not convert itself into testosterone or estrogens in the body.[citation needed]
DHEA and DHEAS are readily available in the United States.
Precautions
Some assert that DHEA should not be supplemented outside specialist centres under careful observation of experts in the field of endocrinology.
Side effects may include:
- Palpitations and other arrhythmias
- extensive growth of body hair, or hirsutism
- Hair loss, especially male pattern baldness
- acne
Contraindication
As DHEAS and DHEA are converted to sex steroids, their use is contraindicated in patients with any cancer that is estrogen or testosterone dependent.[citation needed]
Increasing endogenous production
Regular exercise is known to increase DHEA production in the body.[4][5][6] Caloric restriction has also been shown to increase DHEA in primates.[7]
See also
- Steroid hormones
- Testosterone
- Estrogen
References
- ^ FDA document regading DHEA and SLE
- ^ Kawano H, Yasue H, Kitagawa A, et al. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5.
- ^ Wolkowitz OM, Reus VI, Roberts E, et al. Antidepressant and cognition-enhancing effects of DHEA in major depression. Ann NY Acad Sci. 1995 Dec 29;774:337-9
- ^ Eur J Appl Physiol Occup Physiol 1998 Oct;78(5):466-71
- ^ Eur J Appl Physiol. 2001 Jul;85(1- 2):177-84
- ^ J Gerontol A Biol Sci Med Sci. 2002 Apr;57(4):B158-65
- ^ Exp Gerontol. 2003 Jan-Feb; 38(1-2):35-46
Further reading
- Nutrition through the Life Cycle, Judith E. Brown, ISBN 0-534-58986-3
- Andus T, et al. Patients with refractory Crohn's disease or ulcerative colitis respond to dehydroepiandrosterone: a pilot study. Aliment Pharmacol Ther. 2003 Feb;17(3):409-14.
Arlt W, Callies F, Allolio B. DHEA replacement in women with adrenal insufficiency--pharmacokinetics, bioconversion and clinical effects on well-being, sexuality and cognition. Endocr Res. 2000 Nov;26(4):505-11.
Chang DM, et al. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002 Nov;46(11):2924-7.
Gordon CM, et al. Effects of oral DHEA on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002 Nov;87(11):4935-41.
Hackbert L, Heiman JR. Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. J Womens Health Gend Based Med. 2002 Mar;11(2):155-62.
Hirshman E, et al. The effect of dehydroepiandrosterone (DHEA) on recognition memory decision processes and discrimination in postmenopausal women. Psychon Bull Rev. 2003 Mar;10(1):125-34.
Johannsson G, et al. Low dose DHEA affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab. 2002 May;87(5):2046-52.
Kawano H, et al. DHEA supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5.
Lovas K, et al. Replacement of DHEA in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003 Mar;88(3):1112-8.
Percheron G, et al. Effect of 1-year oral administration of DHEA to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003 Mar 24;163(6):720-7.
Piketty C, et al. Double-blind placebo-controlled trial of oral DHEA in patients with advanced HIV disease. Clin Endocrinol (Oxf). 2001 Sep;55(3):325-30.
Sahelian, R, Borken, S. DHEA and cardiac arrhythmia. Ann Intern Med. 1998 Oct 1;129(7):588.
Sun Y, et al. Treatment of osteoporosis in men using dehydroepiandrosterone sulfate. Chin Med J (Engl). 2002 Mar;115(3):402-4.
Andus T, et al. Patients with refractory Crohn's disease or ulcerative colitis respond to DHEA: a pilot study. Aliment Pharmacol Ther. 2003 Feb;17(3):409-14.
Arlt W, Callies F, Allolio B. DHEA replacement in women with adrenal insufficiency--pharmacokinetics, bioconversion and clinical effects on well-being, sexuality and cognition. Endocr Res. 2000 Nov;26(4):505-11.
Chang DM, et al. DHEA treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002 Nov;46(11):2924-7.
Gordon CM, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002 Nov;87(11):4935-41.
Hackbert L, Heiman JR. Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. J Womens Health Gend Based Med. 2002 Mar;11(2):155-62.
Hirshman E, et al. The effect of dehydroepiandrosterone (DHEA) on recognition memory decision processes and discrimination in postmenopausal women. Psychon Bull Rev. 2003 Mar;10(1):125-34.
Johannsson G, et al. Low dose DHEA affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab. 2002 May;87(5):2046-52.
Kawano H, et al. DHEA supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5.
Lovas K, et al. Replacement of DHEA in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003 Mar;88(3):1112-8.
Percheron G, et al. Effect of 1-year oral administration of DHEA to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003 Mar 24;163(6):720-7.
Piketty C, et al. Double-blind placebo-controlled trial of oral DHEA in patients with advanced HIV disease. Clin Endocrinol (Oxf). 2001 Sep;55(3):325-30.
Sahelian, R, Borken, S. DHEA and cardiac arrhythmia. Ann Intern Med. 1998 Oct 1;129(7):588.
Sun Y, et al. Treatment of osteoporosis in men using DHEA sulfate. Chin Med J (Engl). 2002 Mar;115(3):402-4.
External links
- The DHEA Debate: A critical review of experimental data at lef.org (Published 2004){This link is to the web equivalent of an infomercial. Unreliable sponsored analysis)
- DHEA: Ignore the Hype (Published 1996) at Quackwatch
- DHEA Hormone Replacement at benbest.com
- What the Skeptic's Dictionary has to say on DHEA
- Nair K, Rizza R, O'Brien P, Dhatariya K, Short K, Nehra A, Vittone J, Klee G, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall D, Melton L, Smith G, Khosla S, Jensen M (2006). "DHEA in elderly women and DHEA or testosterone in elderly men". N Engl J Med 355 (16): 1647-59. PMID 17050889. link
- DHEA Supplement by Ray Sahelian, M.D. http://www.raysahelian.com/dhea.html
Whyfiles
Pubmed | Vitamin | 디에이치이에이
Ligandome